Research Group Solovei
Arrangement of chromatin within the nucleus is crucial for transcription regulation and for establishing of cellular identity during differentiation. In the nucleus, separate chromosomes merge into a seamless chromatin mass but euchromatin (EC) and heterochromatin (HC) segregate from each other, which is highly important for proper functioning of the nucleus. (Solovei et al. 2016, PubMed).
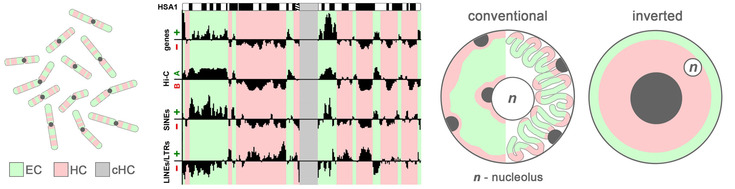
In our group we study mechanisms of EC and HC segregation, including chromosome folding and tethering to the nuclear scaffolding structures. One of our favorite models are nuclei of rod photoreceptors in nocturnal retinas with atypical EC and HC arrangement. In conventional nuclei, EC is localized in the nuclear interior and HC at the nuclear periphery. In nocturnal rods, positions of EC and HC are inverted: all HC is concentrated in the nuclear center, whereas EC forms a thin peripheral shell. We have shown that the central heterochromatin core functions as a microlens reducing light scattering in thick nocturnal retinas and thus facilitates vision in darkness (Solovei et al. 2009, PubMed; Subramanian et al. 2019, PubMed). Studies of inverted rod nuclei helped us to uncover mechanisms of chromatin folding and segregation within conventional nuclei and thus helped to understand general principles of functional nuclear organization – see more:
1. Nuclear inversion is disadvantageous
2. Conventional nuclear organization is established and maintained by tethering heterochromatin to the nuclear envelope by two major mechanisms
3. Chromatin inversion is a natural state of compartment organization
4. Building the nucleus is a self-organizing and predetermined process
The second main direction of our research is spatial organization of transcribed eukaryotic genes. Given the relatively small sizes of mammalian genes and the limited resolution of light microscopy, the structure and spatial arrangement of a single transcribed gene are still poorly understood. Recently, we made use of several long highly expressed mammalian genes and demonstrated that they form Transcription Loops with polymerases moving along the loops and carrying nascent RNAs undergoing co-transcriptional splicing (Mirny & Solovei 2021, PubMed; Leidescher et al 2022, PubMed). See the explanatory Video-1 and Video-2.
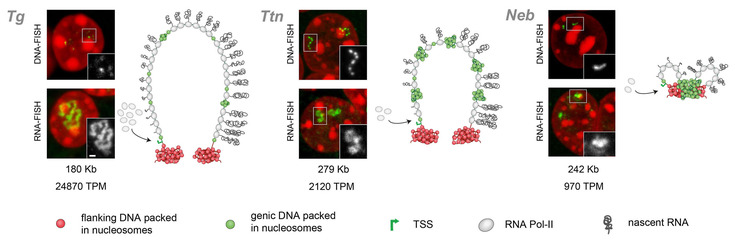
This finding rules out a popular hypothesis about eukaryotic transcription occurring in so called Transcription factories with immobilized polymerases and genes reeling through them. We showed that transcription loops dynamically modify their harboring loci and extend into the nuclear interior suggesting an intrinsic stiffness of these structures. Both experimental evidence and polymer modeling support the hypothesis that transcription loop stiffness is caused by the dense decoration of transcribed genes with multiple voluminous nascent RNPs.

